CroíValve, a pioneering medical device company innovating a better way to help more patients suffering from Tricuspid Regurgitation (TR), was awarded funding from Horizon Europe’s European Innovation Council (EIC) Accelerator Programme.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250702306192/en/
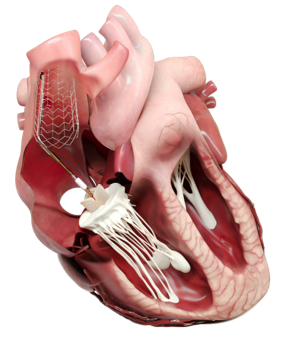
DUO™ Transcatheter Tricuspid System
Part of the EU’s Horizon Europe 2021-2027 Research and Innovation Programme, the EIC Accelerator Programme provides transformational funding to high-potential, high-risk start-ups, scale-ups and subject matter experts. This award includes grant funding of €2.5 million, combined with an equity investment of €10 million in CroiValve’s next financing. The latest competition included 959 applications, with only 40 awards across 16 countries. Almost one third of the selected companies are led by a woman and CroiValve are incredibly proud to be one of this group.
“Securing European Innovation Council (EIC) funding is instrumental in expanding clinical validation of the DUO™ System, a novel minimally invasive, transcatheter device to treat severe+ tricuspid regurgitation. There is a significant unmet clinical need to treat the heterogeneous patient population who are not suitable for first generation transcatheter tricuspid devices. It is CroiValve’s mission to innovate a better way to help more patients suffering with this disease and we are honoured to have EIC support for this journey,” said Lucy O’Keeffe, CEO of CroíValve.
More than 4 million people in Europe and the US suffer from tricuspid regurgitation, a severe heart condition that occurs when the tricuspid valve in the right side of the heart fails to close properly. This results in blood being pumped backwards into the heart causing debilitating symptoms. It affects over 4 million people in Europe and the U.S. and is associated with significant morbidity and reduced life expectancy.
The DUOTM System is a novel transcatheter heart valve that preserves the patient’s native anatomy while treating tricuspid regurgitation. It works in tandem with the native tricuspid valve to restore valve function while leaving the patient’s right heart and native valve apparatus untouched through an innovative anchoring mechanism. Designed to treat a broad patient population, the DUOTM System accommodates the heterogeneity of patient anatomies while avoiding contact with critical structures in the right heart which could lead to complications.
The DUOTM System is also designed to enable a straightforward procedure and reduce the procedural complexity seen with other devices. The procedure is less reliant on expert intraprocedural imaging, enabling a short learning curve with predictable and stable outcomes. The DUO™ System is currently under clinical investigation through the TANDEM II Study, a multicenter, prospective early feasibility study including centers in Poland and the US.
About CroíValve
CroíValve is a clinical stage medical device company focused on the development of a novel transcatheter device for the treatment of tricuspid regurgitation with Research, Development and Operations based in Ireland and Clinical, Regulatory and Marketing based in the United States.
Caution: The DUOTM System is an investigational device and not for sale in any geography.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250702306192/en/
© Business Wire, Inc.
Aviso legal :
Este comunicado de imprensa não é um documento produzido pela AFP. A AFP não será responsável por este conteúdo. Para mais informações, por favor entre em contato com as pessoas ou entidades mencionadas no comunicado.
